Tryptase Prevention of Internal Blood Clots
Life-threatening systemic anaphylaxis occurs when too many IgE-sensitized MCs in the body simultaneously degranulate when they encounter the appropriate antigen. While the adverse roles of MCs and their mediators in severe allergic reactions have been known for decades, the conservation of MCs for more than 500 million years of evolution (Wong et al., Biochem. Biophys. Res. Commun. 2014;415:341) (view Wong et al.) was strong circumstantial evidence that these immune cells were essential for our survival. In support of this conclusion, no human has been found that lacks MCs. The tetramer‑forming tryptases (collectively known as hTryptase-β) encoded by the adjacent TPSAB1 and TPSB2 genes on human chromosome 16p13.3 are the major constituents of the secretory granules of human MCs, and the presence of this family of serine proteases in the serum and/or plasma often is used to identify those with mastocytosis or allergic individuals who have undergone MC-dependent anaphylaxis.
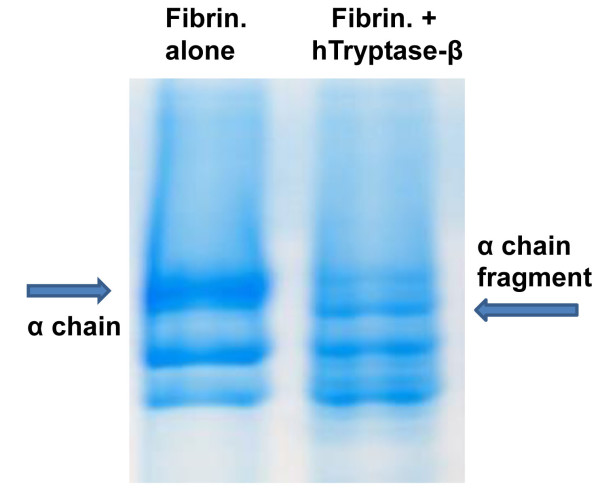
Fig. 2. The α chain of fibrinogen is a preferred target of hTryptase-β. Fibrinogen consists of three differentially sized chains (designated as the α, β, and γ chains). The protein’s α chain was preferentially cleaved when this plasma protein was incubated for 15 min with recombinant hTryptase-β at an ~100:1 substrate:enzyme ratio (right lane) (from Prieto-Garcia et al., J. Biol. Chem. 20012;287:7834).
These tryptases are stored in the secretory granules of human MCs ionically bound to heparin- and chondroitin sulfate E-containing serglycin proteoglycans. In mice, the correspondingTpsb2 and Tpsab1 genes encode the tryptases that are more commonly known as mouse MC protease (mMCP) -6 and mMCP-7 because they were the sixth and seven proteases identified in mouse MCs. Mouse and human MCs also express transmembrane tryptase/tryptase-γ/Prss31 which is encoded by the TPSG1 gene that also resides within the tryptase genomic locus in the two species. Due to their C-terminal membrane-spanning domains, mouse and human PRSS31 are preferentially retained on the outer leaflet of the plasma membrane of activated MCs, thereby enabling MCs to communicate with T cells and other cells that they physically contact in tissues.
In 1997 (Huang et al., J. Biol. Chem. 1997;272:31885) (view Huang et al), we generated recombinant mMCP-7 and showed that the α chain of fibrinogen is a preferred substrate of this MC tryptase in vitro and in vivo. In 2012 (Prieto-Garcia et al., J. Biol. Chem. 20012;287:7834) (view Prieto-Garcia et al), we showed that fibrinogen also is highly susceptible to degradation by hTryptase‑β and mMCP‑6, and that Lys575 is a preferred cleavage site in the protein’s α chain (see Figure 2). Because cutaneous mouse MCs store substantial amounts of tryptase•heparin complexes in their secretory granules, the passive cutaneous anaphylaxis reaction was induced in the skin of transgenic mice that lacked both mMCP-6 and mMCP-7. In support of our in vitro data, fibrin deposits were markedly increased in the skin of the tryptase-deficient mice six hours after IgE‑sensitized animals were given the relevant antigen (see Figure 3). This did not occur in treated wild-type (WT) mice or transgenic mice that lacked heparin. Whether or not the tryptase PRSS31 also participates in blood coagulation in humans and mice has not yet been determined.
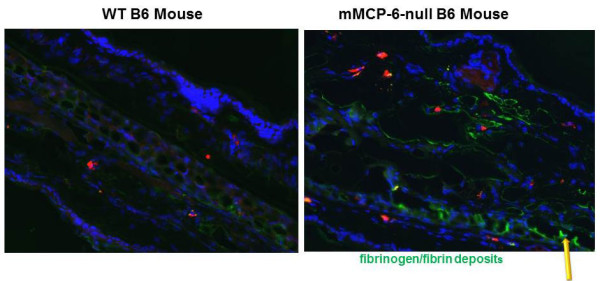
Fig. 3. The tetramer-forming tryptase mMCP-6 prevents fibrin deposition in the MC-dependent passive cutaneous anaphylaxis (PCA) reaction. IgE was injected into the ears of a WT mouse (left panel) and a mMCP-6-null mouse (right panel). The next day, the relevant antigen was injected into both mice. Six hours later, the animals were sacrificed and processed for immunohistochemistry using fluorescein-labeled anti-mouse fibrinogen antibody (green color; yellow arrow). As noted, much more immunoreactive fibrinogen/fibrin was present in the ears of the mMCP-6-null mouse after the PCA reaction had been induced (from Prieto-Garcia et al., J. Biol. Chem. 20012;287:7834).
Fibrinogen is a major constituent of the edema fluid that accumulates in tissues when MCs degranulate. Our discovery that mouse and human tetramer-forming tryptases destroy fibrinogen before this prominent plasma protein can be converted to fibrin in tissues changes the paradigm as to how MCs hinder fibrin deposition and blood coagulation internally. Because of the adverse consequences of fibrin deposits and platelet clots in tissues as occurs in strokes (a leading cause of death worldwide), our data explain why no hTryptase-β-null human has been identified, why mice and humans lack a circulating protease inhibitor that rapidly inactivates these tetramer-forming tryptases, and why mammals have two genes that encode these functionally redundant enzymes. Finally, our data explain why some women have menstrual-like bleeding shortly after they experience a severe anaphylactic event, and why some mastocytosis patients who have an excess of hTryptase-β+ MCs in their body often have excessive bleeding of their skin and gastrointestinal tract (Prieto-Garcia et al., Immunol. Allergy Clin. North Am. 2014;34:263) (view Prieto-Garcia et al).