Beneficial Role of Mast Cell Tryptases in Bacterial Infections
Sepsis is caused by the immune system’s response to a serious infection, most commonly bacteria. Although sepsis is a more serious health problem in the underdeveloped world, those who had to take penicillin or another antibiotic to prevent them from developing sepsis are keenly aware of the importance of our ability to combat even common bacterial strains like E. coli. The role of the MC’s tetramer-forming tryptases in pathogen defense has been reviewed (McNeil et al., J. Biol. Chem. 2007;282:20785) (view McNeil et al.). In 1996, two groups noted that MC‑deficient W/Wv mice cannot combat bacterial infections of their lungs and peritoneal cavity effectively. Neutrophils are the primary cells in the body that kill bacteria. W/Wv mice have fewer numbers of neutrophils in their circulation relative to WT mice. Nevertheless, their diminished ability to combat bacteria was found to be a consequence of fewer MCs rather than fewer neutrophils because the adoptive transfer of mMCP-6+ mouse bone marrow-derived MCs into these mice could rescue their anti-bacteria deficiency. While it was initially concluded in those 1996 studies that widely expressed tumor necrosis factor-α was the key MC-derived factor needed to control bacterial infections, that conclusion was subsequently shown to be incorrect. It therefore became apparent that the relevant anti-bacteria factors had to be more restricted to MCs.
In 1990, we showed that the MCs in the peritoneal cavity contain substantial amounts of mMCP-6 (Reynolds et al., Proc. Natl. Acad. Sci. USA 1990;87:3230) (view Reynolds et al.). In order to evaluate the biologic significance of that finding, we generated recombinant mMCP-6 and its human ortholog hTryptase-β.The administration of just 0.3 nmol of purified recombinant mMCP‑6 (Huang et al., J. Immunol. 1998;160:1910) (view Huang et al.) or hTryptase‑β (Huang et al., J. Biol. Chem. 2001;276:26276) (view Huang et al.) into the peritoneal cavities or lungs of WT or W/Wv mice resulted in a pronounced local neutrophilia. We then showed that we could increase the ability of W/Wv mice to combat a K. pneumoniae infection of their peritoneal cavity if these MC‑deficient mice were given recombinant tryptase twenty four hours before they were infected.
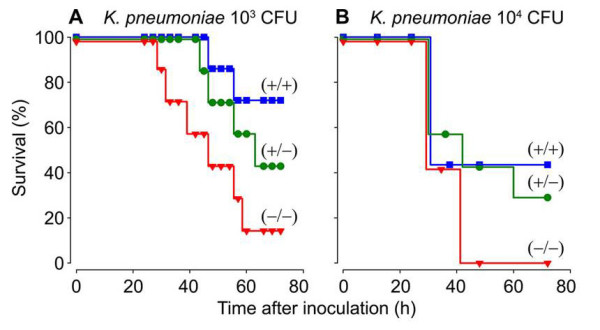
Fig. 4. Survival of WT (+/+), mMCP-6-null (-/-), and heterozygotes (+/-) mice after infection with K. pneumoniae. Whether the three populations of mice were given 1,000 or 10,000 bacteria, fewer mMC-6-null mice could survive the infection sixty hours later (from Thakurdas et al., J. Biol. Chem. 2007;282:20809).
The importance of the MC’s tetramer-forming tryptases in bacterial infections was conclusively shown in 2007 when my coworkers and I generated a transgenic mouse line that could not express either mMCP-6 or mMCP-7 (Thakurdas et al., J. Biol. Chem. 2007;282:20809) (view Thakurdas et al). As shown in that study and Figure 4, these tryptase-null mice quickly died when their lungs were infected with K. pneumonia. The presence of increased numbers of the pathogen in their blood documented that these tryptase-null mice were experiencing sepsis due to an inability to rapidly recruit neutrophils into their infected peritoneal cavity (see Figure 5).
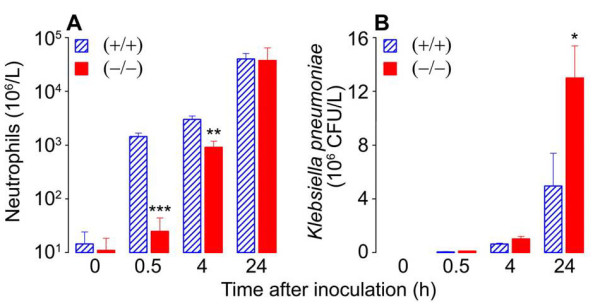
Fig. 5. Neutrophil recruitment (A) and bacterial load (B) during K. pneumoniae infection of WT (+/+) and mMCP-6-null (-/-) mice. Neutrophils are the primary cells in the body that kill bacteria. Since bacteria double ~20 min it is imperative that neutrophils are quickly recruited from the blood to the site of bacterial infection. Because the tetramer-forming tryptase mMCP-6 is needed to induce the rapid accumulation of neutrophils into the infection site (A), sepsis is more pronounced in mMCP-6-null mice (B) (from Thakurdas et al., J. Biol. Chem. 2007;282:20809).
Others showed that the expression of the mMCP-6 gene was highly dependent on microphthalmia-associated transcription factor (MITF). In support of our data, MITF-null mice also had a diminished ability to combat bacteria.
Because exposure of cultured human endothelial cells to mature mMCP-6 or hTryptase β (but not their inactive zymogens) resulted in a substantial increase in the levels of interleukin 8 mRNA and protein, the accumulated data suggested that enzymatically active mMCP-6 and hTryptase-β play beneficial indirect roles in bacterial infections by inducing bystander cells in inflammatory sites to release substantial amounts of neutrophil-specific chemotactic factors that ultimately induce the extravasation of these bactericidal granulocytes into the site to control the infection.